Group 4: Sciences
• Biology HL/SL
• Chemistry HL/SL
• Computer Science HL/SL
• Physics HL/SL
• Environmental Ststems and Societies SL*
*The course Environmental Systems and Societies may count either as a Group 3 or as a Group 4 subject.
The Nature of the Subject:
Biology is the study of the phenomena of life. Biological scientists (researchers, teachers and students) observe living systems and organisms, ask questions, and propose explanations for those observations. IB Biology (SL & HL) covers key topics including the molecules that are present in living organisms and the life processes that are characteristic of all living organisms. The four basic biological concepts that form the backbone of this subject are: structure and function, universality versus diversity, equilibrium within systems, and evolution. Our knowledge of the biological world is based on the scientific enterprise of asking questions and testing hypotheses. In addition to the study of theory issues, Biology students (SL & HL) are also involved in practical investigations and project work.
Programme of Study:
All SL students follow a common core and then study one additional options material.
Part of the course is spent on practical work (experiments) that is assessed and prepares for the personal investigation that takes place at the end of year 1. Additionally all students participate in an interdisciplinary project that takes place in June of the first year.
Core (SL + HL)
Cells
Morecular Biology
Genetics
Ecology
Evolution and Biodiversity
Human Physiology
Additional Higher Level
Nucleic Acids
Metabolism Cell Respiration and Photosynthesis
Plant Biology
Genetics and Evolution
Animal Physiology
Options
Neurobiology and Behavior
Biotechnology and bioinformatics
Ecology and Conservation
Human Physiology
Αssessment:
Students on this course are assessed as follows:
External assessment (written papers, 3 hours) - 80%
The written examinations are in the form of three papers at both Standard and Higher Level.
For Standard Level students
Paper 1 20%
30 multiple-choice questions on the core.
Paper 2 40%
Section A: one data-based question and several short-answer questions on the core
Section B: one extended-response question on the core (from a choice of two).
Paper 3 20%
Several short-answer questions in experimental work described in the syllabus and in the one option.
For Higher Level students
Paper 1 20%
40 multiple-choice questions core and AHL.
Paper 2 36%
Section A: one data-based question and several short-answer questions on the core and the AHL (all compulsory)
Section B: two extended-response questions on the core and the AHL (from a choice of three).
Paper 3 24%
Several short-answer questions in experimental work described in the syllabus and in the one option.
Internal assessment 20%
The internal assessment consists of an interdisciplinary project (Group 4 project) as well as a mixture of short- and long-term investigations. Students at Standard Level have to spend 40 hours on carrying out practical work, while at Higher Level that is 60 hours. Of these 40/60 hours of practical work, 10 hours can be allocated towards the Group 4 project.
Environmental Systems and Societies SL
The Nature of the Subject:
Environmental systems and societies is a trans disciplinary subject that aims to associate experimental sciences with individuals and societies. The course aims to promote an understanding of environmental issues and processes, providing the theoretical background on which students build their knowledge and form their own perspectives, based on their critical awareness of issues and an appreciation of the controversy linked to those issues. Students are encouraged to understand and evaluate environmental concepts and issues in a holistic approach that will allow them to form well-reasoned arguments. Topics covered in this subject include a range of pressing environmental issues such as pollution, global warming, and conservation of biodiversity, to name a few. Both a local as well as an international dimension is considered in the context of such issues. Students are encouraged to work with, process, present, as well as discuss available or collected data in an appropriate scientific manner.
Programme of Study:
The Environmental Systems and Societies (SL) course is offered as a 2-year course, covering the following topics:
Systems and models
The ecosystem and Ecology
Conservation and biodiversity
Water, food production systems and societies
Soil systems and societies
Atmospheric systems and societies
Climate change and energy production
Human systems and resource use
Assessment:
Students on this course are assessed as follows:
External assessment (written papers, 3 hours) - 80%
Paper 1 - 1 hour 25%
(Paper 1 is made up of a case study presented in a resource booklet and a number of short-answer questions)
Paper 2 - 2 hours 50%
(Paper 2 consists of two sections A and B.
Section A involves short answer questions).
In Section B students have to answer two structured essay questions from a choice of four).
Internal assessment - 10 hours - 25%
Students participate in an interdisciplinary project (Group 4 project) and a number of short- and long-term investigations. At the end of the first year they decide on a topic, plan the investigation, perform the experiment and write a lab report. This is the Internal assessment assessed internally and moderated externally.
The performance in internal assessment is judged against the following six assessment criteria Identifying the context, Planning, Results/analysis/conclusion, Discussion/evaluation, Applications and communication and earns in total 30 marks.
Planning - Pl
Data collection and processing - DCP
Discussion, evaluation and conclusion - DEC
Personal skills - PS
Computer Science SL/HL
Computer science requires an understanding of the fundamental concepts of computational thinking as well as knowledge of how computers and other digital devices operate.
“Computer science is no more about computers than astronomy is about telescopes.” - Edsger Dijkstra
Topics Taught
The topics that must be studied in both SL and HL course, including some practical work, are:
• Topic 1: System fundamentals
• Topic 2: Computer organization
• Topic 3: Networks
• Topic 4: Computational thinking, problem-solving and programming
And the extension for the HL course includes the following topics:
• Topic 5: Abstract data structures
• Topic 6: Resource management
• Topic 7: Control
All students will follow Option D: Object Oriented Programming, with the HL students following a more in-depth analysis of the OOP principles.
Assessment
All students and will need to deliver a project, namely the Internal Assessment, which includes both coding a solution, as well as a 2000-word documentation of the overall process of developing it.
External assessment is based on two papers for SL and HL students, whereas HL students only are assessed on a third paper, which is based on a pre-defined case study.
Physics
What is Physics?
Physics is the study of systems from the very small, like electrons or quarks, to the very large, the Universe itself!
In order to understand these systems it is often necessary to develop “models”. These may be mechanical, schematic, computational or mathematical.
What do i need to study Physics?
There is no getting away from the fact that Physics is a mathematical subject. Anyone contemplating studying the subject to this level will need to be a competent mathematician.
You will need to be able to re-arrange equations, solve linear and quadratic and simultaneous equations, resolve vectors using simple trigonometry and be able to deal with the simple trigonometric functions. You will also need to be able to plot graphs and interpret them. You will also need practical skills. You will have to design experiments to test hypotheses, carry out investigations and learn to be critical of your own work and discuss its reliability.
However, above all a physicist needs imagination. To put yourself inside an atomic nucleus or imagine what a galaxy looks like, to suspend your belief and consider the implications of high speed travel or the uncertainties of the behaviour of small particles and photons requires considerable vision.
Physicists are good at solving problems because they can reduce them to their bare essentials and so you need an analytical and critical mind and be able to approach problems in a logical way.
You will develop these skills over the five terms of the course. It will be challenging but rewarding and, hopefully, stimulating and good fun!
Syllabus Outline
For the Higher Level candidates must study:
Measurement and Uncertainties
Mechanics
Thermal Physics
Wave Phenomena
Electro-Magnetism
Quantum Physics and Nuclear Physics
Energy production
Digital technology (HL only)
Standard level candidates study the same topics but in less depth. Their option topics are in the core of the Higher level syllabus.
In addition there are some options.
Imaging
Engineering Physics
Astro-Physics
Relativity
Assessment
The Assessment is carried out as follows:
Written exam 5 hours 80%
Paper 1 - 1 hour 20%
Multiple choice paper on the core topics
Paper 2 - 2¼ hours 40%
Short-answer and extended-response questions on the core and AHL material
Paper 3 - 1¼ hours 20%
Section A: one data-based question and several short-answer questions on experimental work.
Section B: short-answer and extended-response questions from one option.
Internal Assessment 20%
Project and investigation work.
This will include an individual investigation involvig experimental work and an interdisciplinary group investigation called the group IV project.
Chemistry
The Subject
Chemistry is the study of substances, what they are made of, how they interact and the role they play in life. Alchemy, the ancient ancestor of modern Chemistry, was said to be the art of the transformation of simple materials into precious metals. Even though this concept appears mythical it reflects the dimensions of modern Chemistry. In fact it still is the science of transformation since it helps us explain, understand and forge the world around us.
Moreover Chemistry is a fundamental science and hence it is a prerequisite for many other courses in higher education, such as Medicine, Biology, Engineering and Environmental sciences.
Aims
The IB Chemistry course is designed to increase students’ knowledge and understanding of the subject, their ability to solve problems and at the same time enhance their practical and investigative abilities; to deal with abstract and theoretical material; enrich their knowledge and understanding of the social and economic importance of Chemistry; foster independent study, practical work and research. As an intensive pre-University course it also aims into pupils’ preparation for Chemistry related studies and employment.
Qualifications necessary
It is assumed that everyone coming to study IB Chemistry will have, at least, an above average performance in their science subjects to date. Pupils who have only achieved a lower grade may find some aspects of the course difficult. A certain amount of mathematical knowledge is required but this is not excessive and students will be taught to the required standard.
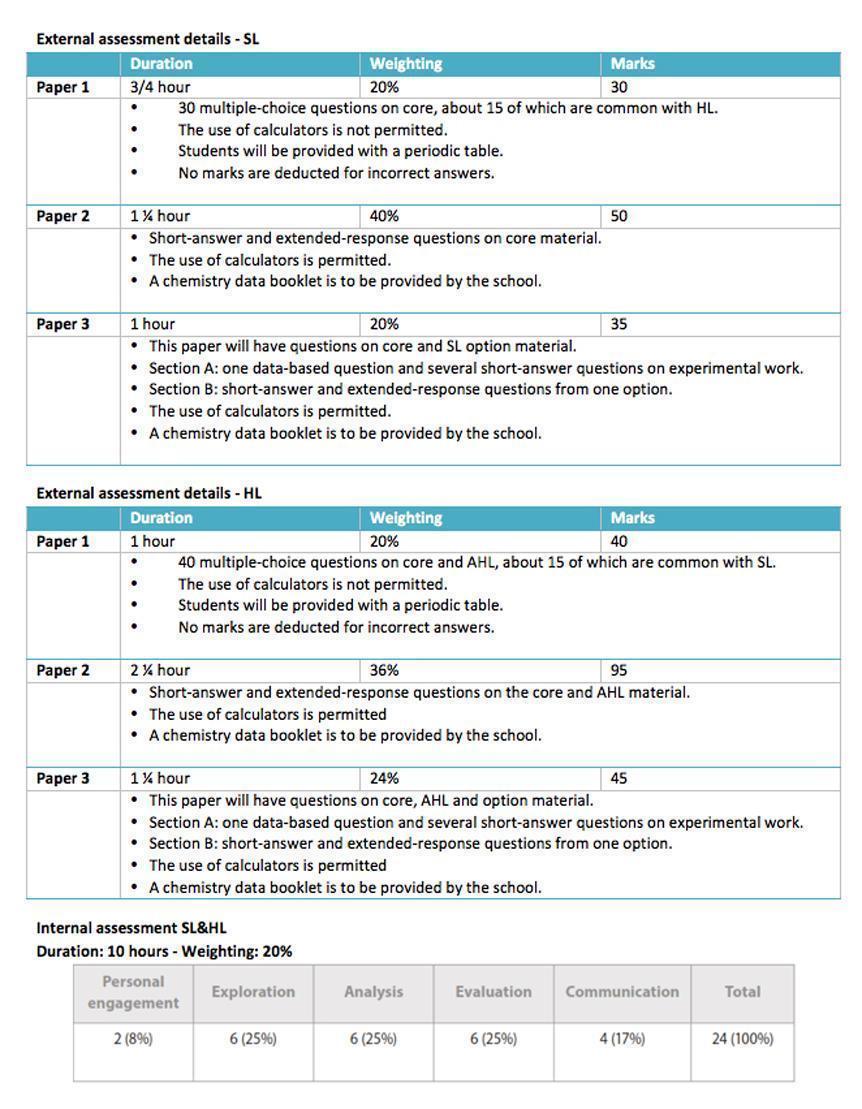
The IB Chemistry Course
The IB Chemistry course is offered at both Standard and Higher Level. The course itself is split into four basic sections: the subject specific core, the additional Higher Level material, the optional units (two of which are obligatory) and the Group 4 project.
The core material provides a solid foundation on which the remainder of the course is built. The additional Higher Level work explores some of the work already covered in the core topics but in a much greater depth and difficulty as well. There are a variety of optional units available and those offered will be specific to the expertise of the staff.
Syllabus outline
The syllabus comprises of the following units, many of which do not differ, at least in title, from the Greek Curriculum.
Quantitative chemistry - Equilibrium
Atomic Structure - Acids and bases
Periodicity - Oxidation and reduction
Bonding - Organic chemistry
Energetics - Measurement and data processing
Kinetics
What differentiates utterly the IB Chemistry course is the way it is taught, approached and assessed. At least 1/3 of the curriculum (40 & 60 hours for SL and HL respectively) is investigated through hands on practicals executed independently by the students (naturally under supervision and help by their teachers). Moreover the course is inviting students to cultivate their own perception on the subjects by essay writing, creative science projects and independent research in library and literature resources.
Assessment
The course assessment, in class, follows the official IB assessment. Students are graded 80% by their performance in tests and examinations, and 20% by their performance in the Internal Assessment.
It is needless to state that aspects like proper conduct, responsible and ethical behavior, may not be quantifiable as above but are surely assessed as well.
The course is assessed in the end of the two year program by written examination, which is worth 80% of the total mark (each paper is worth 20%, 36% and 20% respectively). The remaining 20% is made up from the internal assessment of practical skills and the Group 4 project. The practical skills are assessed both internally (by the teachers) and externally by IBO by submitting of the students’ scientific exploration project.
The exams specifications are listed below.